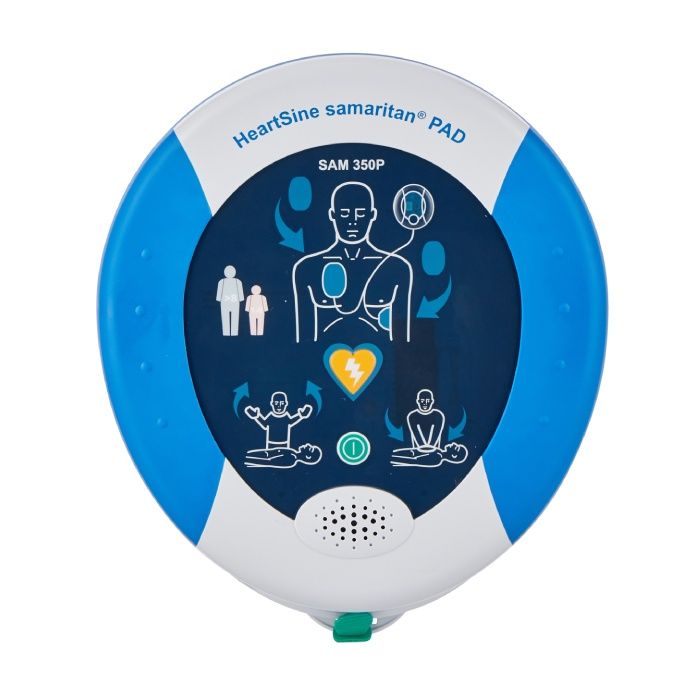
HeartSine samaritan PAD 350P Semi Automatic
In stock
Simplistic and easy to use, the HeartSine samaritan PAD 350P has been designed for use by both trained and untrained responders alike.
Key Defibrillator Features
- Portable
- Lightweight
- IP56 rating
- Advanced SCOPE biphasic technology
- User-friendly interface
- Easy-to-understand visual and voice prompts
- Simple, two-button operation
- System Status Ready indicator flashes to indicate the unit is operational
Interested in renting this defibrillator? For more information click here
The HeartSine samartian PAD 350P defibrillator's compact and easy-to-use design makes it suitable for use by trained and untrained users. Equipped with visual and verbal aids, users can feel confident in the treatment they're delivering, regardless of their training history.
What's Included?
- 1 x HeartSine samaritan PAD 350P Semi Automatic Defibrillator
- 1 x HeartSine samaritan Adult PAD PAK - electrode pads and battery in one easy cartridge
- 1 x HeartSine samaritan Soft Carry Case
- 1 x AED Armor Rescue Kit
- 1 x Quick Reference Instruction Card
- 8 Year Manufacturer's Warranty
Additional products available for this unit
- HeartSine samaritan Paediatric PAD-PAK - electrode pads and battery in one easy cartridge
Read More here: Forward Hearts Program
More Information
For further information about this product, get in touch with a member of our team on 0161 776 7422.
| Connectivity | None |
|---|---|
| FDA Approved | Yes |
| Warranty | 8 Year Manufacturer's Warranty |
| IP Rating | 56: the AED is protected from limited dust ingress and protected from high pressure water jets from any direction |
| Defib Brand | HeartSine |
| Semi/Fully Automatic | Semi Automatic |
| Battery Life | 4 years |
| On Screen ECG | No |
| Energy Delivery | Escalating |
| Manual Override | No |
| Memory | 90 mins |
| Pad Life | Up to 4 years |
| Dimensions | H: 20cm x W: 18.4cm x D: 4.8cm |
| Product Weight | 1.1kg |
| Dangerous Goods | No |
| Icon | Label | Description | Type | Size | Download |
|---|---|---|---|---|---|

|
HeartSine samaritan PAD User Manual | Defibrillator user manual | 3.5 MB | Download |
More Information
For further information about this product, get in touch with a member of our team on 0161 776 7422.
How is the HeartSine samaritan PAD 350P Semi-Automatic easy to use?
The user-friendly device provides simple visual and audio cues to assist the rescuer through resuscitation. This includes CPR, which is a critical link in the chain of survival, with only an ON/OFF button (and the shock button on the SAM 350P). The operation is basic and uncomplicated.
The device is always prepared. The system status-ready indicator illuminates that the entire system is functioning and ready for use. Every week, the unit does a self-check.
Is the HeartSine samaritan PAD 350P Semi-Automatic suitable for use on children?
The HeartSine samaritan PAD 350P Semi-Automatic is suitable for use on children and adults. Children younger than 8 years or weighing less than 55lbs require a smaller joule output than the HeartSine samaritan Adult PAD PAK that is included with your purchase. There is the option to purchase the Paediatric Pad Pak.
What is the charging time in the HeartSine samaritan PAD 350P Semi-Automatic?
Time is crucial during a sudden cardiac arrest. The sooner treatment is provided, the higher the chance of survival. The charging time for a samaritan PAD 350P defibrillator is typically 150J in 8 seconds and 200J in 12 seconds. This means life-saving defibrillation will be delivered between 8-12 seconds.
How is the HeartSine samaritan PAD 350P Semi-Automatic low-cost ownership?
Low-cost ownership means your device does not cost much money to maintain.
With a four-year shelf life, the Pad-Pak, which includes a set of electrode pads and a battery in one cartridge, represents considerable savings over other defibrillators requiring separate battery and electrode replacements.